AXIM Biotechnologies, Inc. (OTC: AXIM), an innovative company focused on the research, development and production of hemp-based pharmaceutical, nutraceutical and cosmetic products with an emphasis on the well-being of their customers, announced today that it has secured the down payment on land to build a brand new, state-of-the-art facility on 6,000 square meters of land located at the industrial estate of Stichtsekant, in the city of Almere, The Netherlands. AXIM Biotech’s future high-tech factory will be located on the corner of Fort de Gagel and Fort Blauwkape.
Holding exclusive rights to the world’s first patented cannabinoid release chewing gum and, with it, plans to conduct clinical trials for pain and spasticity associated with Multiple Sclerosis (MS) set in the EU, AXIM Biotech is leading the life sciences industry with hemp-based innovations. AXIM’s focus lies in developing unique and proprietary delivery mechanisms for the introduction of cannabinoids (i.e. THC, CBD, CBG, CBN, etc.) and finding solutions for conditions for which there is currently no effective treatment including MS, spasticity, pain, Parkinson’s disease, Alzheimer’s disease/dementia, ADHD (attention deficit hyperactivity disorder), PTSD, autism, RLS (restless leg syndrome), glaucoma, IBD, IBS and Crohn’s disease.
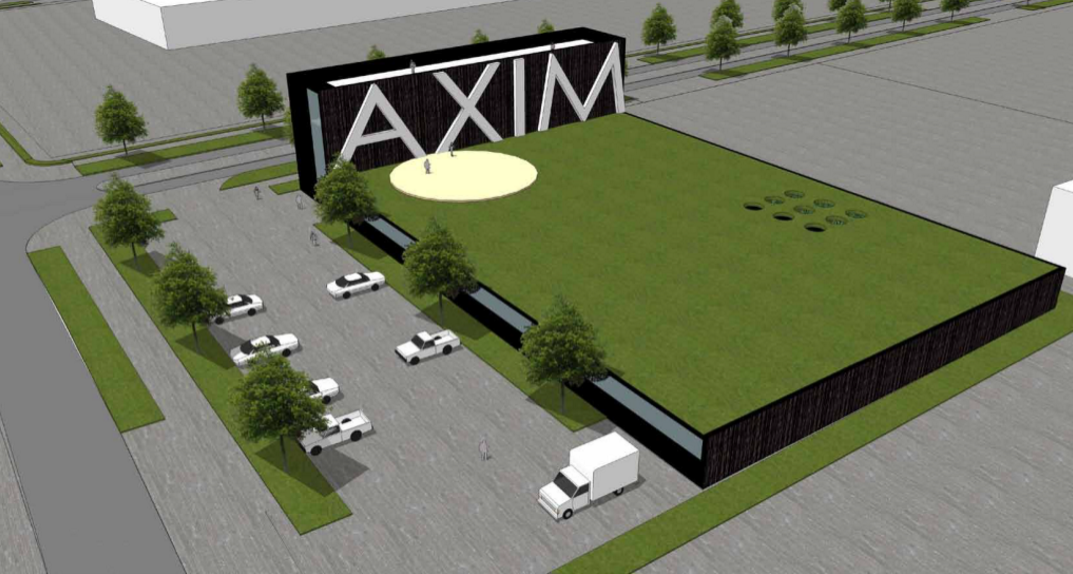
AXIM Biotechnologies, Inc. Future Manufacturing Facility in Almere, The Netherlands.
With the first payment secured, AXIM Biotech will now begin to work with the builder and architects on detailed building and engineering plans with respect to permit applications and subsequent approvals. After the final purchase of the land, AXIM Biotech will break ground on construction.
“We are extremely grateful to the government of the province of Flevoland for allowing AXIM Biotech to establish manufacturing in Almere. The factory plans are compliant with the highest European and International standards and will produce unique pharmaceutical and nutraceutical products – all from agricultural hemp.” – Dr. George E. Anastassov, MD – Chief Executive Officer of AXIM Biotech
AXIM Biotechnologies’ state-of-the-art facility will feature a clean laboratory zone, storage areas, office and technical rooms as well as manufacturing facility furnishings. AXIM’s global manufacturing hub in Almere will have the capacity to process raw materials and manufacture the Company’s innovative hemp-based pharmaceutical, nutraceutical, and consumer products, which are all based on their own intellectual property. Project design and feasibility have begun and AXIM is targeting the completion of construction by 2017.
“This is an exciting time for life sciences as AXIM establishes a fully ‘green’ agricultural hemp-based biotech manufacturing facility. At AXIM, the hemp manufacturing byproducts will be used and result in a net negative carbon footprint that is beneficial to the environment.” – Lekhram Changoer – Chief Technical Officer of AXIM BioTech
Research shows that the industrial hemp plant can produce more than 25,000 products. Focusing on innovations in health and the environment, AXIM Biotech’s Almere facility will include manufacturing in the following product categories:
- Functional foods
- Nutraceuticals
- Pharmaceuticals
- Cosmetics
- Clean energy
Given that this facility is overseas and will be state-of-the-art, it will be interested to see how AXIM is able to impact the medical hemp markets globally and here in the United States. While completion of the facility isn’t expected until 2017, AXIM has already developed or acquired the intellectual property that could pole-vault them to the forefront of the medical hemp industry. Perhaps the most unique skew to the consumer being the time-released cannabis chewing gum, which would be the first of its kind.
What do you think about AXIM’s new facility and medical hemp development? How about the time-released cannabis gum? Join the conversation and comment below!




