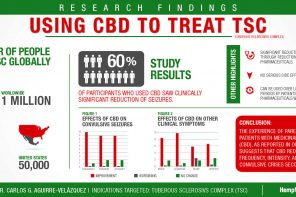
New cannabinoid medicine can be successfully administered via a topical gel lens into the eye, a new InMed-University of British Columbia study reports.
“The InMed-UBC study is the first-ever to report the use of a nanoparticle hydrogel for drug delivery into the eye, resulting in direct drug uptake by the cornea and lens,” according to a release.
Eye drops and similar formulations are washed away with blinking, so on average less than five percent of the drug reaches its target tissue.
“Total drug delivered using this hydrogel nanoparticle formulation was three-times higher than the standard control formulation,” according to a release.
InMed, a biopharmeceutical company, describes its proprietary hydrogel delivery method as having “unique rheological characteristics permitting it to form a thin, uniform coating (a gel-like lens) over the cornea through blinking of the eyelid. This lens holds the drug in place and allows for trans-corneal absorption of the drug, which can then diffuse within the eye to the retina.”
Eric A. Adams, InMed president and CEO, said “This study offers the first tangible proof of our ability to successfully identify specific drug targets using our bioinformatic assessment tools, manufacture a cannabinoid using our proprietary biosynthesis process, load the drug into a patented, target specific formulation and deliver effective dose levels to a target tissue.”
This company-sponsored research study was recently published in the peer reviewed Journal of Controlled Release (JCR), entitled “A stimulus-responsive, in situ forming, nanoparticle-laden hydrogel for ocular drug delivery.”
The study is part of InMed’s biosynthesis program in which they are seeking to treat Epidermolysis Bullosa, a group of inherited connective tissue diseases that share a common manifestation of extremely fragile skin that blisters or tears easily from friction or trauma. Internal organs and bodily systems can also be affected by EB. EB is an orphan disease with no currently approved treatments and has a significant unmet medical need.
Cannabinoid-based medicine INM-750 will potentially be the first therapy designed and developed specifically to modulate disease activity and to alleviate symptoms in EB.
With over 90 cannabinoids yet to be discovered, biopharmaceutical companies are racing to identify these medicinal compounds and their uses with new biotechnology.
With their first-of-its-kind biosynthesis program, InMed scientists used e-coli to produce a purified version of a new non-CBD cannabinoid compound, and then put the compound into InMed’s topical gel solution.
While the company has not released additional information on the cannabinoid compound, they have stated it is not CBD-based.
InMed specializes in successfully producing cannabinoids using genetically engineered microorganisms, by introducing the cannabis plant’s metabolic pathways into bacteria and yeast. “These molecules can be functionalized further to produce any of the 90+ “downstream” cannabinoids found naturally in the cannabis plant,” according to a release.
InMed views its biosynthesis discoveries as having huge potential for industrial-scale manufacturing of cannaboinoid medicines.
Adams said, “The biosynthesis process opens up access to drug discovery and therapeutic use of all 90+ cannabinoids, most of which occur in only trace amounts in the plant and cannot be extracted in an economical fashion.”